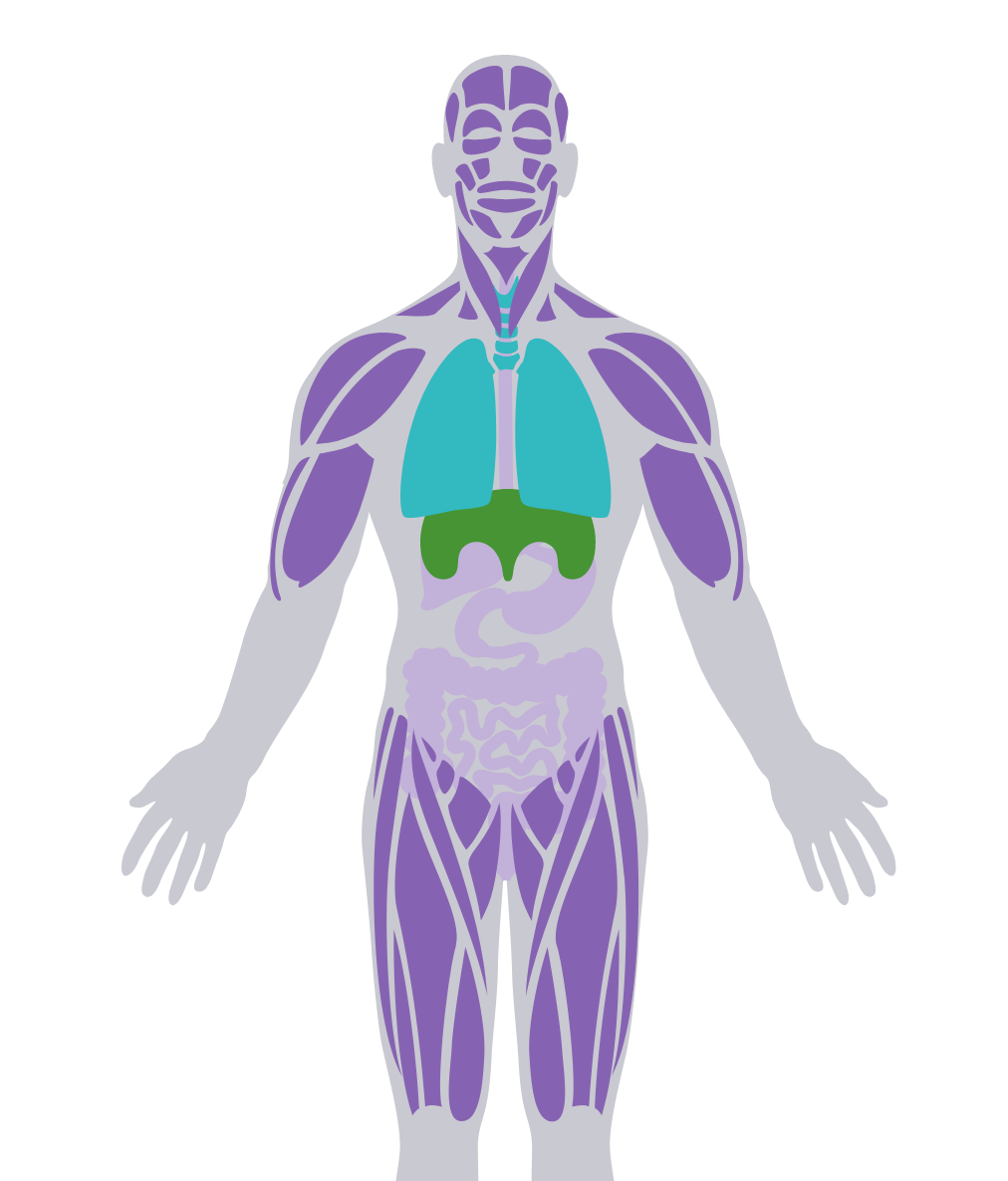
LOPD is a degenerative neuromuscular disorder that affects walking and breathing ability1,2
Late-onset Pompe disease (LOPD) can present anytime from infancy to adulthood. Signs and symptoms of LOPD can include:
-
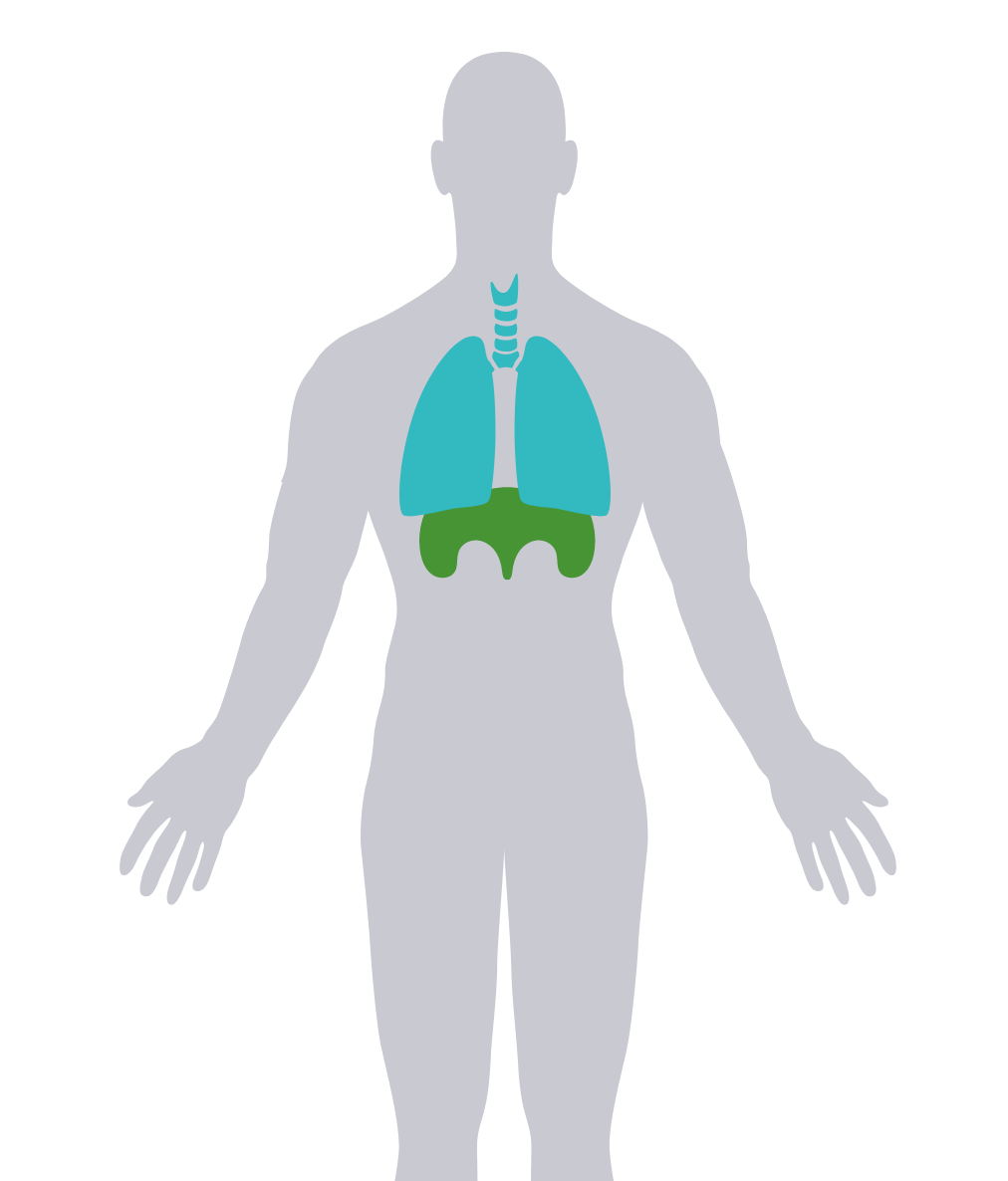
LOPD can affect respiratory muscles, including the diaphragm, and can be progressive.3
- Respiratory insufficiency
- Shortness of breath
- Orthopnea
- Sleep disordered breathing
- Impaired cough
- Respiratory infections/pneumonia

LOPD can affect respiratory muscles, including the diaphragm, and can be progressive.3
- Respiratory insufficiency
- Shortness of breath
- Orthopnea
- Sleep disordered breathing
- Impaired cough
- Respiratory infections/pneumonia

-
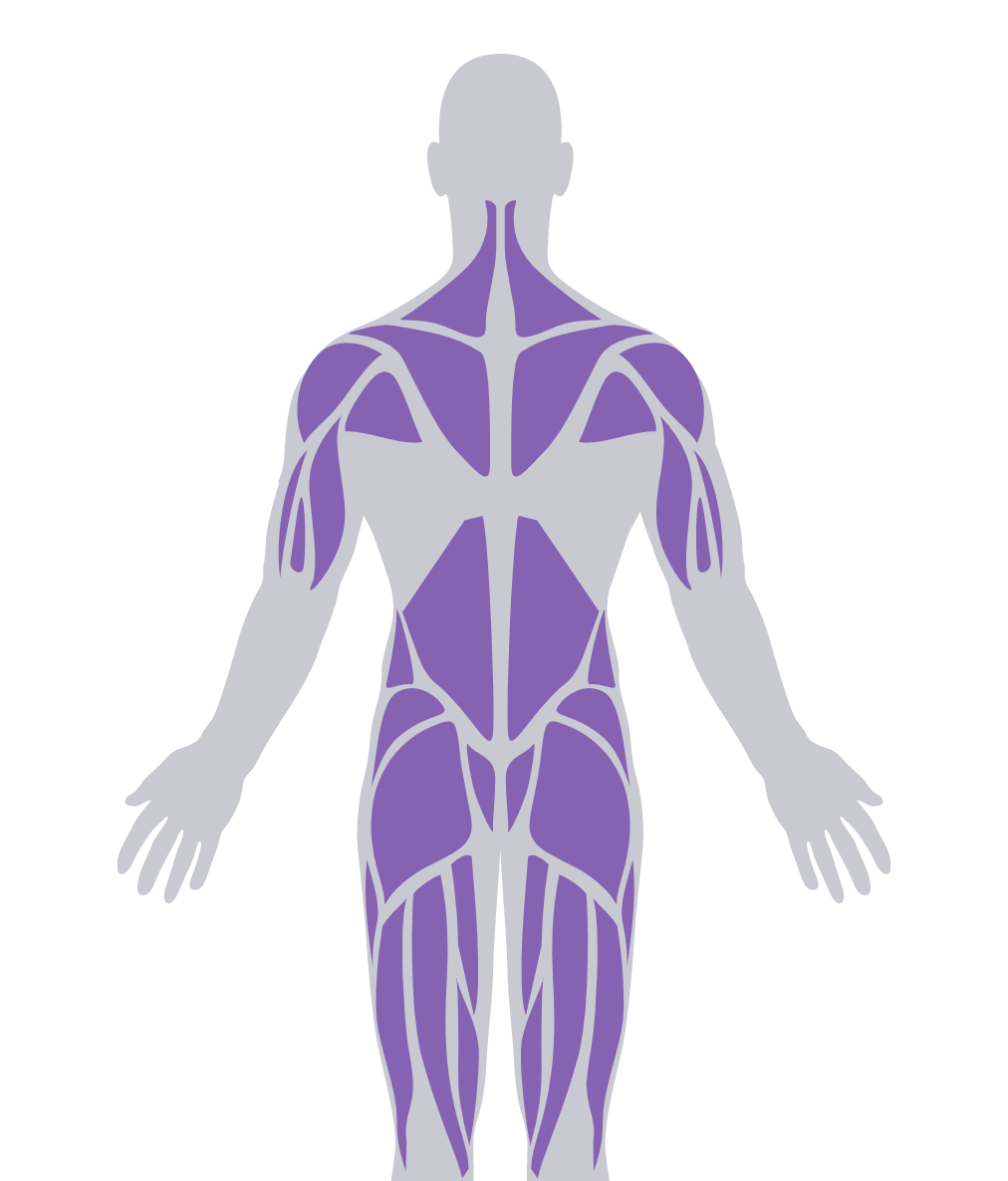
Progressive myopathy is the most common feature, which can have pronounced impact on proximal muscles of the lower extremities.4
- Progressive muscle weakness
- Gait abnormalities
- Gower’s sign
- Frequent falls/trips
- Scapular winging
- Elevated serum CK

Progressive myopathy is the most common feature, which can have pronounced impact on proximal muscles of the lower extremities.4
- Progressive muscle weakness
- Gait abnormalities
- Gower’s sign
- Frequent falls/trips
- Scapular winging
- Elevated serum CK

-
Other signs and symptoms include2,4,5:
- Difficulty swallowing/chewing
- Gastroesophageal reflux disease
- Diarrhea
- Elevated liver enzymes: aspartate aminotransferase and alanine aminotransaminase
- Exercise intolerance
- Fatigue
- Morning headaches

Other signs and symptoms include2,4,5:
- Difficulty swallowing/chewing
- Gastroesophageal reflux disease
- Diarrhea
- Elevated liver enzymes: aspartate aminotransferase and alanine aminotransaminase
- Exercise intolerance
- Fatigue
- Morning headaches


The Doctor Discussion Guide can be shared with patients to help you have a productive conversation at their next appointment.
Testing for LOPD2,4,6-8
If you see the signs of LOPD, consider your testing options.
ㅤ
SUSPECT POMPE
(run a Pompe-specific test)
ㅤ
GAA enzyme activity testing

BLOOD

Reduced GAA enzyme activity can confirm Pompe diagnosis
OR
Genetic confirmation


GAA pathogenic variants identified via GAA gene sequencing
SUSPECT NEUROMUSCULAR DISORDERS (NMDs)
(run a genetic panel that covers multiple genes and NMDs simultaneously)

BLOOD OR SALIVA SAMPLE


GAA pathogenic variants identified
The above flowchart is intended to serve as an example.
|
WARNING: SEVERE HYPERSENSITIVITY REACTIONS, INFUSION-ASSOCIATED REACTIONS, and RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS Hypersensitivity Reactions including Anaphylaxis Patients treated with NEXVIAZYME have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily available during NEXVIAZYME administration. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, NEXVIAZYME should be discontinued immediately and appropriate medical treatment should be initiated. In patients with severe hypersensitivity reaction, a desensitization procedure to NEXVIAZYME may be considered. Infusion-Associated Reactions (IARs) Patients treated with NEXVIAZYME have experienced severe IARs. If severe IARs occur, consider immediate discontinuation of NEXVIAZYME, initiation of appropriate medical treatment, and the benefits and risks of readministering NEXVIAZYME following severe IARs. Patients with an acute underlying illness at the time of NEXVIAZYME infusion may be at greater risk for IARs. Patients with advanced Pompe disease may have compromised cardiac and respiratory function, which may predispose them to a higher risk of severe complications from IARs. Risk of Acute Cardiorespiratory Failure in Susceptible Patients Patients susceptible to fluid volume overload, or those with acute underlying respiratory illness or compromised cardiac or respiratory function for whom fluid restriction is indicated may be at risk of serious exacerbation of their cardiac or respiratory status during NEXVIAZYME infusion. More frequent monitoring of vitals should be performed during NEXVIAZYME infusion. |
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Including Anaphylaxis: See Boxed WARNING. Prior to NEXVIAZYME administration, consider pretreating with antihistamines, antipyretics, and/or corticosteroids. The risks and benefits of readministering NEXVIAZYME following severe hypersensitivity reaction (including anaphylaxis) should be considered. If a mild or moderate hypersensitivity reaction occurs, the infusion rate may be slowed or temporarily stopped.
Infusion-Associated Reactions: See Boxed WARNING. IARs may still occur in patients after receiving pretreatment. If mild or moderate IARs occur regardless of pretreatment, decreasing the infusion rate or temporarily stopping the infusion may ameliorate the symptoms.
Risk of Acute Cardiorespiratory Failure in Susceptible Patients: See Boxed WARNING.
ADVERSE REACTIONS
The most common adverse reactions (>5%) were headache, fatigue, diarrhea, nausea, arthralgia, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia and urticaria.
INDICATION
NEXVIAZYME (avalglucosidase alfa-ngpt) is indicated for the treatment of patients 1 year of age and older with late-onset Pompe disease [lysosomal acid alpha-glucosidase (GAA) deficiency].
Please see full Prescribing Information for complete details, including Boxed WARNING.
References: 1. Llerena Junior JC, Nascimento OJ, Oliveira AS, et al. Arq Neuropsiquiatr. 2016;74(2):166-176. 2. American Association of Neuromuscular & Electrodiagnostic Medicine. Muscle Nerve. 2009;40(1):149-160. 3. Mellies U, Lofaso F. Respir Med. 2009;103:477-484. 4. Kishnani PS, Steiner RD, Bali D, et al. Genet Med. 2006;8(5):267-288. 5. Data on file. Genzyme Corporation. 6. Pompe Disease Diagnostic Working Group. Mol Genet Metab. 2008;93(3):275-281. 7. Xue Y, Ankala A, Wilcox W, et al. Genet Med. 2015;17(6):444-451. 8. LGMD Genetic Testing Program. Muscular Dystrophy Association. Published April 6, 2018. Accessed October 28, 2022. https://www.mda.org/disease/limb-girdle-muscular-dystrophy/diagnosis/lgmd-genetic-testing-program.
|
WARNING: SEVERE HYPERSENSITIVITY REACTIONS, INFUSION-ASSOCIATED REACTIONS, and RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS Hypersensitivity Reactions including Anaphylaxis Patients treated with NEXVIAZYME have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily available during NEXVIAZYME administration. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, NEXVIAZYME should be discontinued immediately and appropriate medical treatment should be initiated. In patients with severe hypersensitivity reaction, a desensitization procedure to NEXVIAZYME may be considered. Infusion-Associated Reactions (IARs) Patients treated with NEXVIAZYME have experienced severe IARs. If severe IARs occur, consider immediate discontinuation of NEXVIAZYME, initiation of appropriate medical treatment, and the benefits and risks of readministering NEXVIAZYME following severe IARs. Patients with an acute underlying illness at the time of NEXVIAZYME infusion may be at greater risk for IARs. Patients with advanced Pompe disease may have compromised cardiac and respiratory function, which may predispose them to a higher risk of severe complications from IARs. Risk of Acute Cardiorespiratory Failure in Susceptible Patients Patients susceptible to fluid volume overload, or those with acute underlying respiratory illness or compromised cardiac or respiratory function for whom fluid restriction is indicated may be at risk of serious exacerbation of their cardiac or respiratory status during NEXVIAZYME infusion. More frequent monitoring of vitals should be performed during NEXVIAZYME infusion. |
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Including Anaphylaxis: See Boxed WARNING. Prior to NEXVIAZYME administration, consider pretreating with antihistamines, antipyretics, and/or corticosteroids. The risks and benefits of readministering NEXVIAZYME following severe hypersensitivity reaction (including anaphylaxis) should be considered. If a mild or moderate hypersensitivity reaction occurs, the infusion rate may be slowed or temporarily stopped.
Infusion-Associated Reactions: See Boxed WARNING. IARs may still occur in patients after receiving pretreatment. If mild or moderate IARs occur regardless of pretreatment, decreasing the infusion rate or temporarily stopping the infusion may ameliorate the symptoms.
Risk of Acute Cardiorespiratory Failure in Susceptible Patients: See Boxed WARNING.
ADVERSE REACTIONS
The most common adverse reactions (>5%) were headache, fatigue, diarrhea, nausea, arthralgia, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia and urticaria.
INDICATION
NEXVIAZYME (avalglucosidase alfa-ngpt) is indicated for the treatment of patients 1 year of age and older with late-onset Pompe disease [lysosomal acid alpha-glucosidase (GAA) deficiency].
Please see full Prescribing Information for complete details, including Boxed WARNING.
References: 1. Llerena Junior JC, Nascimento OJ, Oliveira AS, et al. Arq Neuropsiquiatr. 2016;74(2):166-176. 2. American Association of Neuromuscular & Electrodiagnostic Medicine. Muscle Nerve. 2009;40(1):149-160. 3. Mellies U, Lofaso F. Respir Med. 2009;103:477-484. 4. Kishnani PS, Steiner RD, Bali D, et al. Genet Med. 2006;8(5):267-288. 5. Data on file. Genzyme Corporation. 6. Pompe Disease Diagnostic Working Group. Mol Genet Metab. 2008;93(3):275-281. 7. Xue Y, Ankala A, Wilcox W, et al. Genet Med. 2015;17(6):444-451. 8. LGMD Genetic Testing Program. Muscular Dystrophy Association. Published April 6, 2018. Accessed October 28, 2022. https://www.mda.org/disease/limb-girdle-muscular-dystrophy/diagnosis/lgmd-genetic-testing-program.

.png)